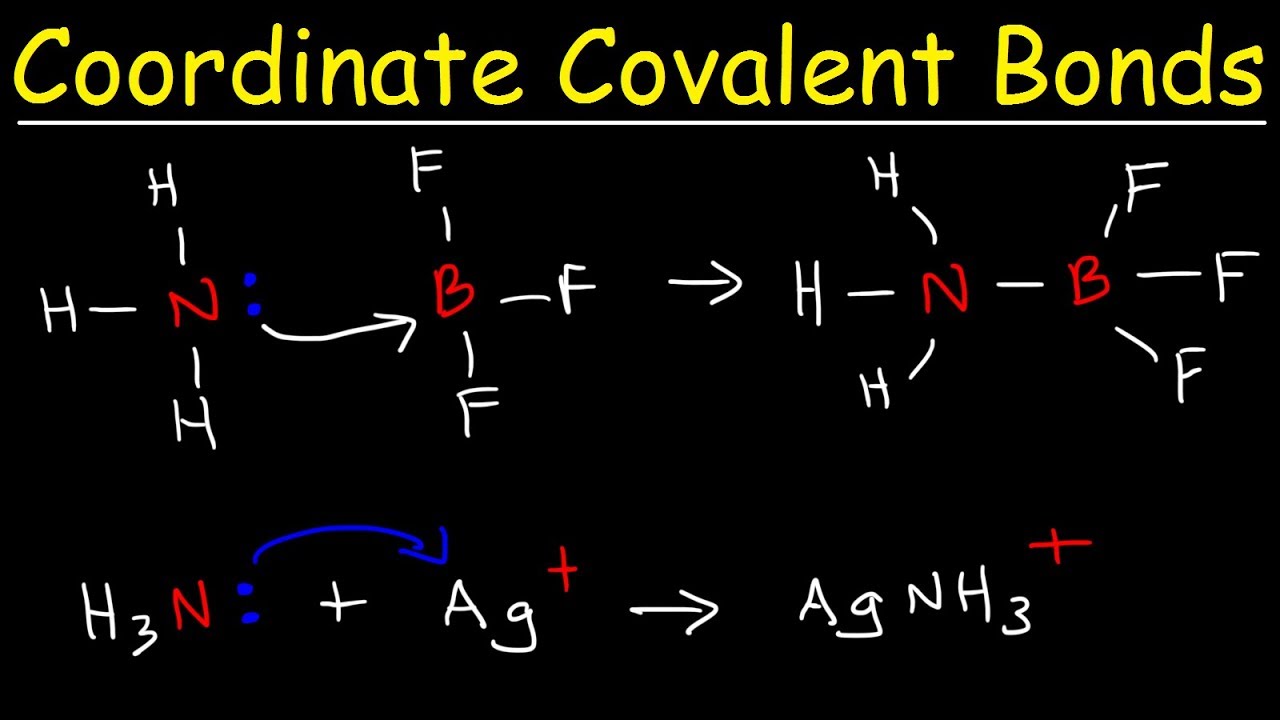
Does Boron Form Covalent Bonds . to say that boron forms only covalent compounds is an oversimplification. Values are given for typical oxidation. boron can form ions but there is some fine print. You won't get monatomic cations like the metals below it. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Hydrogen makes sense because it has only one shell and 2. It can be involved in metallic or even predominantly ionic. covalent radius half of the distance between two atoms within a single covalent bond. hydrogen and boron seem to be the only outliers to the octet rule. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead.
from mungfali.com
It can be involved in metallic or even predominantly ionic. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. hydrogen and boron seem to be the only outliers to the octet rule. covalent radius half of the distance between two atoms within a single covalent bond. to say that boron forms only covalent compounds is an oversimplification. Values are given for typical oxidation. You won't get monatomic cations like the metals below it. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. boron can form ions but there is some fine print.
Covalent Bonding Structure
Does Boron Form Covalent Bonds to say that boron forms only covalent compounds is an oversimplification. covalent radius half of the distance between two atoms within a single covalent bond. Values are given for typical oxidation. You won't get monatomic cations like the metals below it. hydrogen and boron seem to be the only outliers to the octet rule. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. It can be involved in metallic or even predominantly ionic. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. to say that boron forms only covalent compounds is an oversimplification. boron can form ions but there is some fine print. Hydrogen makes sense because it has only one shell and 2. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Does Boron Form Covalent Bonds covalent radius half of the distance between two atoms within a single covalent bond. Hydrogen makes sense because it has only one shell and 2. hydrogen and boron seem to be the only outliers to the octet rule. boron can form ions but there is some fine print. instead of forming a metallic lattice with delocalized. Does Boron Form Covalent Bonds.
From www.tec-science.com
Covalent bonding tecscience Does Boron Form Covalent Bonds you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. It can be involved in metallic or even predominantly ionic. hydrogen and boron seem to be the only outliers to the octet rule. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. You won't get monatomic. Does Boron Form Covalent Bonds.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Does Boron Form Covalent Bonds Values are given for typical oxidation. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. boron can form ions but there is some fine print. to say that boron forms only covalent compounds is an oversimplification. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain. Does Boron Form Covalent Bonds.
From asgburange.blogspot.com
BORON FOR UNDERGRADUATES Does Boron Form Covalent Bonds It can be involved in metallic or even predominantly ionic. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. Hydrogen makes sense because it has only one shell and 2. covalent radius half of the distance between two atoms within a single covalent bond. Values are given for typical oxidation. instead of forming. Does Boron Form Covalent Bonds.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples Does Boron Form Covalent Bonds Values are given for typical oxidation. to say that boron forms only covalent compounds is an oversimplification. boron can form ions but there is some fine print. covalent radius half of the distance between two atoms within a single covalent bond. You won't get monatomic cations like the metals below it. Hydrogen makes sense because it has. Does Boron Form Covalent Bonds.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Does Boron Form Covalent Bonds hydrogen and boron seem to be the only outliers to the octet rule. Hydrogen makes sense because it has only one shell and 2. boron can form ions but there is some fine print. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Values are given for typical oxidation.. Does Boron Form Covalent Bonds.
From chem.libretexts.org
Structure & Bonding Chemistry LibreTexts Does Boron Form Covalent Bonds It can be involved in metallic or even predominantly ionic. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. boron can form ions but there is some fine print. Values are given for typical oxidation. to say that boron forms only covalent compounds is an oversimplification. You won't get monatomic cations like the. Does Boron Form Covalent Bonds.
From www.nagwa.com
Question Video Understanding How Ammonia Molecules Bond Boron Does Boron Form Covalent Bonds to say that boron forms only covalent compounds is an oversimplification. covalent radius half of the distance between two atoms within a single covalent bond. You won't get monatomic cations like the metals below it. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Values are given for typical. Does Boron Form Covalent Bonds.
From www.echemi.com
SiliconBoron Covalent Bond ECHEMI Does Boron Form Covalent Bonds You won't get monatomic cations like the metals below it. Values are given for typical oxidation. covalent radius half of the distance between two atoms within a single covalent bond. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. to say that boron forms only covalent compounds is an. Does Boron Form Covalent Bonds.
From phys.org
Unprecedented formation of a boronboron covalent bond opens a new Does Boron Form Covalent Bonds to say that boron forms only covalent compounds is an oversimplification. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. Values are given for typical oxidation. boron can form ions but there is some fine print. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain. Does Boron Form Covalent Bonds.
From www.pinterest.com
Coordinate Covalent Bond Dative Bond Boron Atom, Ionic Compound Does Boron Form Covalent Bonds You won't get monatomic cations like the metals below it. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. covalent radius half of the distance between two atoms within a single covalent bond. hydrogen and boron seem to be the only outliers to the octet rule. It can be. Does Boron Form Covalent Bonds.
From www.slideserve.com
PPT Chemistry Bonding PowerPoint Presentation, free download ID Does Boron Form Covalent Bonds boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. to say that boron forms only covalent compounds is an oversimplification. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. It can be involved in metallic or even predominantly ionic. hydrogen. Does Boron Form Covalent Bonds.
From www.youtube.com
Is BF3 (Boron trifluoride) Ionic or Covalent/Molecular? YouTube Does Boron Form Covalent Bonds boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Values are given for typical oxidation. boron can form ions but there is some fine print. to say that boron forms only. Does Boron Form Covalent Bonds.
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds Does Boron Form Covalent Bonds boron can form ions but there is some fine print. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Hydrogen makes sense because it has only one shell and 2. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. You won't get monatomic cations like. Does Boron Form Covalent Bonds.
From slideplayer.com
Lewis Dot Structures. ppt download Does Boron Form Covalent Bonds Values are given for typical oxidation. to say that boron forms only covalent compounds is an oversimplification. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Hydrogen makes sense because it has only one shell and 2. instead of forming a metallic lattice with delocalized valence electrons, boron forms. Does Boron Form Covalent Bonds.
From littleeagles.edu.vn
Boron Valence Electrons Boron Valency (B) With Dot Diagram Does Boron Form Covalent Bonds Values are given for typical oxidation. Hydrogen makes sense because it has only one shell and 2. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. You won't get monatomic cations like the metals below it. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. . Does Boron Form Covalent Bonds.
From mavink.com
Covalent Bond Types Does Boron Form Covalent Bonds hydrogen and boron seem to be the only outliers to the octet rule. you might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. Hydrogen makes sense because it has only one shell and 2. to say. Does Boron Form Covalent Bonds.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Does Boron Form Covalent Bonds covalent radius half of the distance between two atoms within a single covalent bond. Hydrogen makes sense because it has only one shell and 2. Values are given for typical oxidation. boron commonly makes only three covalent bonds, resulting in only six valence electrons around the \(\ce{b}\) atom. you might perhaps wonder why boron doesn't form ionic. Does Boron Form Covalent Bonds.